Forschung
AG PD Dr. Louisa Steines
The average lifetime of a transplanted kidney is limited to about 10-15 years. Immunological factors are the primary cause of allograft failure. Antibody-mediated rejection (AMR), when donor specific antibodies (DSA) damage the allograft, is the most common cause of kidney transplant failure beyond the first year. In addition, other transplanted organs, such as hearts and lungs, are also prone to attack by DSA. However, there is no effective treatment for this condition, particularly when AMR takes on a chronic course. Strategies to prevent formation or resurgence of DSA are therefore paramount. To achieve this, a thorough understanding of the mechanisms leading to DSA production in organ transplantation are essential.
Dr Steines’s group is interested in understanding the mechanisms of T and B cell activation in the context of transplantation. We are using patient samples, human in vitro cellular assays and a transplant model in rodents to study cellular and humoral alloresponses. We are particularly interested in T-B cell interactions and the regulation of the germinal center reaction, as a source of high affinity alloantibodies. The aim of Dr Steines’s research is to identify novel targets for more effective immunosuppression in transplant medicine.
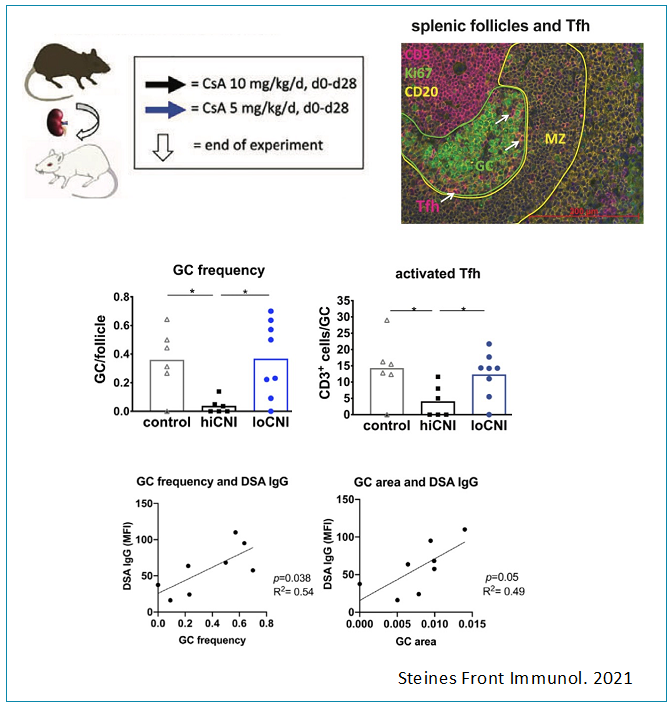
We have studied the dynamics of germinal centers under various conditions after kidney transplantation in a translational rat model, demonstrating a correlation of DSA and germinal center expansion and frequency. Furthermore, we have established a novel in vitro model of T follicular helper cell differentiation based on T-B bidirectional activation, which we are using to study the effects of novel pharmacological interventions.
A major focus of our group’s work is the role of transcriptional repressor Bcl6 in organ transplantation. Bcl6 is essential for the formation of specialized T follicular helper cells and germinal center B cells. In a DFG-funded project, our group is exploring the potential of small molecule Bcl6 inhibitors to inhibit humoral alloresponses. The project is associated to the TRR 374 collaborative research consortium.
-
- PD Dr med Louisa Steines, MD BSc, group leader
- Stefanie Ellmann, lab technician
- Julius Friedmann, doctoral student (medicine)
- Myriam Bouziani, doctoral student (medicine), DTG travel grant recipient
- N.K., doctoral student (medicine), doctoral grant recipient (medicine)
-
- C.C. Baan, Transplantation Institute Rotterdam, the Netherlands
- K. Amann, Department of Nephropathology, University Hospital Erlangen
-
DFG-Project Funding to Dr Steines
-
- Steines, L. Not so marginal…the unexpected role of marginal zone B cells in alloanitbody formation. Transplantation 2024 Mar 22. Doi: : 10.1097/TP.0000000000004988. Online ahead of print.
- Friedman, J., Schuster, A., Reichelt-Wurm, S., Banas, B., Bergler, T., Steines, L. Serum IL-6 predicts risk of kidney transplant failure independently of immunological risk Transpl Immunol. 2024 Mar 27:84:102043.doi: 10.1016/j.trim.2024.102043.Online ahead of print.
- Steines, L., Scharf, M., Hoffmann, P., Schuster, A.M., Banas, B., Bergler, T. Monitoring B cell alloresponses in rats. J Immunol Methods 2021 Dec 29;501:113212. doi: 10.1016/j.jim.2021.113212.
- Siska, P.J., Decking, S.M., Babl, N., Matos, C., Bruss, C., Singer, K., Klitzke, J., Schön, M., Simeth, J., Köstler, J., Siegmund, H., Ugele, I., Paulus, M., Dietl, A., Kolodova, K., Steines, L., Freitag, K., Peuker, A., Schönhammer, G., Raithel, J., Graf, B., Geismann, F., Lubnow, M., Mack, M., Hau, P., Bohr, C., Burkhardt, R., Gessner, A., Salzberger, B., Wagner, R., Hanses, F., Hitzenbichler, F., Heudobler, D., Lüke, F., Pukrop, T., Herr, W., Wolff, D., Spang, R., Poeck, H., Hoffmann, P., Jantsch, J., Brochhausen, C., Lunz, D., Rehli, M., Kreutz, M., Renner, K. Metabolic imbalance of T-cells in severe COVID-19 is hallmarked by basigin expression and mitigated by dexamethasone. J Clin Invest. 2021 Nov 15;131(22):e148225. doi:10.1172/JCI148225.PMID: 34779418
- Steines, L., Poth, H., Schuster, A., Amann, K., Banas, B., Bergler, T. Disruption of Tfh:B Cell Interactions Prevents Antibody-Mediated Rejection in a Kidney Transplant Model in Rats: Impact of Calcineurin Inhibitor Dose. Front Immunol. 2021 May 31;12:657894. doi: 10.3389/fimmu.2021.657894. eCollection 2021.
- Steines, L., Poth, H., Herrmann, M., Schuster, A., Banas, B., Bergler, T. B cell activating factor (BAFF) is required for the development of intra-renal tertiary lymphoid organs in experimental kidney transplantation in rats. Int J Mol Sci. 2020 Oct 28;21(21):8045. doi: 10.3390/ijms21218045.
- Steines, L., Poth, H., Schuster, A., Geissler, E. K., Amann, K., Banas, B., Bergler, T. Anti-BAFF treatment interferes with humoral responses in a model of renal transplantation in rats. Transplantation. 2020 Jan;104(1):e16-e22.doi: 10.1097/TP.0000000000002992.
- Kühne, L., Jung, B., Poth, H., Schuster, A., Wurm, S., Rümmele, P., Banas, B., Bergler, T. Renal allograft rejection, lymphocyte infiltration, and de novo donor-specific antibodies in a novel model of non-adherence to immunosuppressive therapy. BMC Immunol 18, 52, doi:10.1186/s12865-017-0236-6 (2017).
- Schuster, A., Jung, B., Hofbauer, J., Kühne, L., Zecher, D., Banas, B., Bergler, T. B-cell activating factor BAFF reflects patients’ immunological risk profile after kidney transplantation. Transpl Immunol. 2017 Dec; 45:35-41.
- Bergler, T., Jung, B., Bourier, F., Kühne, L., Rümmele, P., Wurm, S., Banas B. Infiltration of macrophages in human kidney transplantation correlates with severity of allograft rejection and outcome. PLoS One. 2016 Jun 10; 11 (6): e0156900.